Cellulose
| |
| |
| Identifiers | |
|---|---|
| ChEMBL | |
| ChemSpider |
|
| ECHA InfoCard | 100.029.692 |
| EC Number |
|
| E number | E460 (thickeners, ...) |
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| Properties | |
| (C 6H 10O 5) n | |
| Molar mass | 162.1406 g/mol per glucose unit |
| Appearance | white powder |
| Density | 1.5 g/cm3 |
| Melting point | 260–270 °C; 500–518 °F; 533–543 K (decomposes)[2] |
| none | |
| Thermochemistry | |
Std enthalpy of
formation (ΔfH⦵298) |
−963 kJ/mol[clarification needed] |
Std enthalpy of
combustion (ΔcH⦵298) |
−2828 kJ/mol[clarification needed] |
| Hazards | |
| NFPA 704 (fire diamond) | |
| NIOSH (US health exposure limits): | |
PEL (Permissible)
|
TWA 15 mg/m3 (total) TWA 5 mg/m3 (resp)[2] |
REL (Recommended)
|
TWA 10 mg/m3 (total) TWA 5 mg/m3 (resp)[2] |
IDLH (Immediate danger)
|
N.D.[2] |
| Related compounds | |
Related compounds
|
Starch |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
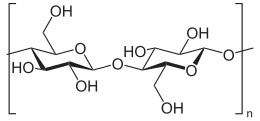
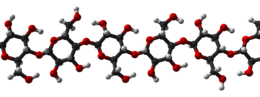
Cellulose is an organic compound with the formula (C
6H
10O
5)
n, a polysaccharide consisting of a linear chain of several hundred to many thousands of β(1→4) linked D-glucose units.[3][4] Cellulose is an important structural component of the primary cell wall of green plants, many forms of algae and the oomycetes. Some species of bacteria secrete it to form biofilms.[5] Cellulose is the most abundant organic polymer on Earth.[6] The cellulose content of cotton fibre is 90%, that of wood is 40–50%, and that of dried hemp is approximately 57%.[7][8][9]
Cellulose is mainly used to produce paperboard and paper. Smaller quantities are converted into a wide variety of derivative products such as cellophane and rayon. Conversion of cellulose from energy crops into biofuels such as cellulosic ethanol is under development as a renewable fuel source. Cellulose for industrial use is mainly obtained from wood pulp and cotton.[6] Cellulose is also greatly affected by direct interaction with several organic liquids.[10]
Some animals, particularly ruminants and termites, can digest cellulose with the help of symbiotic micro-organisms that live in their guts, such as Trichonympha. In human nutrition, cellulose is a non-digestible constituent of insoluble dietary fiber, acting as a hydrophilic bulking agent for feces and potentially aiding in defecation.
History
[edit]Cellulose was discovered in 1838 by the French chemist Anselme Payen, who isolated it from plant matter and determined its chemical formula.[3][11][12] Cellulose was used to produce the first successful thermoplastic polymer, celluloid, by Hyatt Manufacturing Company in 1870. Production of rayon ("artificial silk") from cellulose began in the 1890s and cellophane was invented in 1912. Hermann Staudinger determined the polymer structure of cellulose in 1920. The compound was first chemically synthesized (without the use of any biologically derived enzymes) in 1992, by Kobayashi and Shoda.[13]

Structure and properties
[edit]
Cellulose has no taste, is odorless, is hydrophilic with the contact angle of 20–30 degrees,[14] is insoluble in water and most organic solvents, is chiral and is biodegradable. It was shown to melt at 467 °C in pulse tests made by Dauenhauer et al. (2016).[15] It can be broken down chemically into its glucose units by treating it with concentrated mineral acids at high temperature.[16]
Cellulose is derived from D-glucose units, which condense through β(1→4)-glycosidic bonds. This linkage motif contrasts with that for α(1→4)-glycosidic bonds present in starch and glycogen. Cellulose is a straight chain polymer. Unlike starch, no coiling or branching occurs and the molecule adopts an extended and rather stiff rod-like conformation, aided by the equatorial conformation of the glucose residues. The multiple hydroxyl groups on the glucose from one chain form hydrogen bonds with oxygen atoms on the same or on a neighbour chain, holding the chains firmly together side-by-side and forming microfibrils with high tensile strength. This confers tensile strength in cell walls where cellulose microfibrils are meshed into a polysaccharide matrix. The high tensile strength of plant stems and of the tree wood also arises from the arrangement of cellulose fibers intimately distributed into the lignin matrix. The mechanical role of cellulose fibers in the wood matrix responsible for its strong structural resistance, can somewhat be compared to that of the reinforcement bars in concrete, lignin playing here the role of the hardened cement paste acting as the "glue" in between the cellulose fibres. Mechanical properties of cellulose in primary plant cell wall are correlated with growth and expansion of plant cells.[17] Live fluorescence microscopy techniques are promising in investigation of the role of cellulose in growing plant cells.[18]


Compared to starch, cellulose is also much more crystalline. Whereas starch undergoes a crystalline to amorphous transition when heated beyond 60–70 °C in water (as in cooking), cellulose requires a temperature of 320 °C and pressure of 25 MPa to become amorphous in water.[19]
Several types of cellulose are known. These forms are distinguished according to the location of hydrogen bonds between and within strands. Natural cellulose is cellulose I, with structures Iα and Iβ. Cellulose produced by bacteria and algae is enriched in Iα while cellulose of higher plants consists mainly of Iβ. Cellulose in regenerated cellulose fibers is cellulose II. The conversion of cellulose I to cellulose II is irreversible, suggesting that cellulose I is metastable and cellulose II is stable. With various chemical treatments it is possible to produce the structures cellulose III and cellulose IV.[20]
Many properties of cellulose depend on its chain length or degree of polymerization, the number of glucose units that make up one polymer molecule. Cellulose from wood pulp has typical chain lengths between 300 and 1700 units; cotton and other plant fibers as well as bacterial cellulose have chain lengths ranging from 800 to 10,000 units.[6] Molecules with very small chain length resulting from the breakdown of cellulose are known as cellodextrins; in contrast to long-chain cellulose, cellodextrins are typically soluble in water and organic solvents.
The chemical formula of cellulose is (C6H10O5)n where n is the degree of polymerization and represents the number of glucose groups.[21]
Plant-derived cellulose is usually found in a mixture with hemicellulose, lignin, pectin and other substances, while bacterial cellulose is quite pure, has a much higher water content and higher tensile strength due to higher chain lengths.[6]: 3384
Cellulose consists of fibrils with crystalline and amorphous regions.[22] These cellulose fibrils may be individualized by mechanical treatment of cellulose pulp, often assisted by chemical oxidation or enzymatic treatment, yielding semi-flexible cellulose nanofibrils generally 200 nm to 1 μm in length depending on the treatment intensity.[23] Cellulose pulp may also be treated with strong acid to hydrolyze the amorphous fibril regions, thereby producing short rigid cellulose nanocrystals a few 100 nm in length.[24] These nanocelluloses are of high technological interest due to their self-assembly into cholesteric liquid crystals,[25] production of hydrogels or aerogels,[26] use in nanocomposites with superior thermal and mechanical properties,[27] and use as Pickering stabilizers for emulsions.[28]
Processing
[edit]Biosynthesis
[edit]In plants cellulose is synthesized at the plasma membrane by rosette terminal complexes (RTCs). The RTCs are hexameric protein structures, approximately 25 nm in diameter, that contain the cellulose synthase enzymes that synthesise the individual cellulose chains.[29] Each RTC floats in the cell's plasma membrane and "spins" a microfibril into the cell wall.
RTCs contain at least three different cellulose synthases, encoded by CesA (Ces is short for "cellulose synthase") genes, in an unknown stoichiometry.[30] Separate sets of CesA genes are involved in primary and secondary cell wall biosynthesis. There are known to be about seven subfamilies in the plant CesA superfamily, some of which include the more cryptic, tentatively-named Csl (cellulose synthase-like) enzymes. These cellulose syntheses use UDP-glucose to form the β(1→4)-linked cellulose.[31]
Bacterial cellulose is produced using the same family of proteins, although the gene is called BcsA for "bacterial cellulose synthase" or CelA for "cellulose" in many instances.[32] In fact, plants acquired CesA from the endosymbiosis event that produced the chloroplast.[33] All cellulose synthases known belongs to glucosyltransferase family 2 (GT2).[32]
Cellulose synthesis requires chain initiation and elongation, and the two processes are separate. Cellulose synthase (CesA) initiates cellulose polymerization using a steroid primer, sitosterol-beta-glucoside, and UDP-glucose. It then utilises UDP-D-glucose precursors to elongate the growing cellulose chain. A cellulase may function to cleave the primer from the mature chain.[34]
Cellulose is also synthesised by tunicate animals, particularly in the tests of ascidians (where the cellulose was historically termed "tunicine" (tunicin)).[35]
Breakdown (cellulolysis)
[edit]Cellulolysis is the process of breaking down cellulose into smaller polysaccharides called cellodextrins or completely into glucose units; this is a hydrolysis reaction. Because cellulose molecules bind strongly to each other, cellulolysis is relatively difficult compared to the breakdown of other polysaccharides.[36] However, this process can be significantly intensified in a proper solvent, e.g. in an ionic liquid.[37]
Most mammals have limited ability to digest dietary fibre such as cellulose. Some ruminants like cows and sheep contain certain symbiotic anaerobic bacteria (such as Cellulomonas and Ruminococcus spp.) in the flora of the rumen, and these bacteria produce enzymes called cellulases that hydrolyze cellulose. The breakdown products are then used by the bacteria for proliferation.[38] The bacterial mass is later digested by the ruminant in its digestive system (stomach and small intestine). Horses use cellulose in their diet by fermentation in their hindgut.[39] Some termites contain in their hindguts certain flagellate protozoa producing such enzymes, whereas others contain bacteria or may produce cellulase.[40]
The enzymes used to cleave the glycosidic linkage in cellulose are glycoside hydrolases including endo-acting cellulases and exo-acting glucosidases. Such enzymes are usually secreted as part of multienzyme complexes that may include dockerins and carbohydrate-binding modules.[41]
Breakdown (thermolysis)
[edit]At temperatures above 350 °C, cellulose undergoes thermolysis (also called 'pyrolysis'), decomposing into solid char, vapors, aerosols, and gases such as carbon dioxide.[42] Maximum yield of vapors which condense to a liquid called bio-oil is obtained at 500 °C.[43]
Semi-crystalline cellulose polymers react at pyrolysis temperatures (350–600 °C) in a few seconds; this transformation has been shown to occur via a solid-to-liquid-to-vapor transition, with the liquid (called intermediate liquid cellulose or molten cellulose) existing for only a fraction of a second.[44] Glycosidic bond cleavage produces short cellulose chains of two-to-seven monomers comprising the melt. Vapor bubbling of intermediate liquid cellulose produces aerosols, which consist of short chain anhydro-oligomers derived from the melt.[45]
Continuing decomposition of molten cellulose produces volatile compounds including levoglucosan, furans, pyrans, light oxygenates, and gases via primary reactions.[46] Within thick cellulose samples, volatile compounds such as levoglucosan undergo 'secondary reactions' to volatile products including pyrans and light oxygenates such as glycolaldehyde.[47]
Hemicellulose
[edit]Hemicelluloses are polysaccharides related to cellulose that comprises about 20% of the biomass of land plants. In contrast to cellulose, hemicelluloses are derived from several sugars in addition to glucose, especially xylose but also including mannose, galactose, rhamnose, and arabinose. Hemicelluloses consist of shorter chains – between 500 and 3000 sugar units.[48] Furthermore, hemicelluloses are branched, whereas cellulose is unbranched.
Regenerated cellulose
[edit]Cellulose is soluble in several kinds of media, several of which are the basis of commercial technologies. These dissolution processes are reversible and are used in the production of regenerated celluloses (such as viscose and cellophane) from dissolving pulp.
The most important solubilizing agent is carbon disulfide in the presence of alkali. Other agents include Schweizer's reagent, N-methylmorpholine N-oxide, and lithium chloride in dimethylacetamide. In general, these agents modify the cellulose, rendering it soluble. The agents are then removed concomitant with the formation of fibers.[49] Cellulose is also soluble in many kinds of ionic liquids.[50]
The history of regenerated cellulose is often cited as beginning with George Audemars, who first manufactured regenerated nitrocellulose fibers in 1855.[51] Although these fibers were soft and strong -resembling silk- they had the drawback of being highly flammable. Hilaire de Chardonnet perfected production of nitrocellulose fibers, but manufacturing of these fibers by his process was relatively uneconomical.[51] In 1890, L.H. Despeissis invented the cuprammonium process – which uses a cuprammonium solution to solubilize cellulose – a method still used today for production of artificial silk.[52] In 1891, it was discovered that treatment of cellulose with alkali and carbon disulfide generated a soluble cellulose derivative known as viscose.[51] This process, patented by the founders of the Viscose Development Company, is the most widely used method for manufacturing regenerated cellulose products. Courtaulds purchased the patents for this process in 1904, leading to significant growth of viscose fiber production.[53] By 1931, expiration of patents for the viscose process led to its adoption worldwide. Global production of regenerated cellulose fiber peaked in 1973 at 3,856,000 tons.[51]
Regenerated cellulose can be used to manufacture a wide variety of products. While the first application of regenerated cellulose was as a clothing textile, this class of materials is also used in the production of disposable medical devices as well as fabrication of artificial membranes.[53]
Cellulose esters and ethers
[edit]The hydroxyl groups (−OH) of cellulose can be partially or fully reacted with various reagents to afford derivatives with useful properties like mainly cellulose esters and cellulose ethers (−OR). In principle, although not always in current industrial practice, cellulosic polymers are renewable resources.
Ester derivatives include:
| Cellulose ester | Reagent | Example | Reagent | Group R |
|---|---|---|---|---|
| Organic esters | Organic acids | Cellulose acetate | Acetic acid and acetic anhydride | H or −(C=O)CH3 |
| Cellulose triacetate | Acetic acid and acetic anhydride | −(C=O)CH3 | ||
| Cellulose propionate | Propionic acid | H or −(C=O)CH2CH3 | ||
| Cellulose acetate propionate (CAP) | Acetic acid and propanoic acid | H or −(C=O)CH3 or −(C=O)CH2CH3 | ||
| Cellulose acetate butyrate (CAB) | Acetic acid and butyric acid | H or −(C=O)CH3 or −(C=O)CH2CH2CH3 | ||
| Inorganic esters | Inorganic acids | Nitrocellulose (cellulose nitrate) | Nitric acid or another powerful nitrating agent | H or −NO2 |
| Cellulose sulfate | Sulfuric acid or another powerful sulfating agent | H or −SO3H |
Cellulose acetate and cellulose triacetate are film- and fiber-forming materials that find a variety of uses. Nitrocellulose was initially used as an explosive and was an early film forming material. When plasticized with camphor, nitrocellulose gives celluloid.
Cellulose Ether[54] derivatives include:
| Cellulose ethers | Reagent | Example | Reagent | Group R = H or | Water solubility | Application | E number |
|---|---|---|---|---|---|---|---|
| Alkyl | Halogenoalkanes | Methylcellulose | Chloromethane | −CH3 | Cold/Hot water-soluble[55] | E461 | |
| Ethylcellulose (EC) | Chloroethane | −CH2CH3 | Water-insoluble | A commercial thermoplastic used in coatings, inks, binders, and controlled-release drug tablets,[56] also employed in the production of oleogels and bioplastics[57] | E462 | ||
| Ethyl methyl cellulose | Chloromethane and chloroethane | −CH3 or −CH2CH3 | E465 | ||||
| Hydroxyalkyl | Epoxides | Hydroxyethyl cellulose | Ethylene oxide | −CH2CH2OH | Cold/hot water-soluble | Gelling and thickening agent [58] | |
| Hydroxypropyl cellulose (HPC) | Propylene oxide | −CH2CH(OH)CH3 | Cold water-soluble | filming properties, coating properties, pharmaceuticals, cultural heritage restoration, electronic applications, cosmetic sector [59] [60] [61] [62] [63] | E463 | ||
| Hydroxyethyl methyl cellulose | Chloromethane and ethylene oxide | −CH3 or −CH2CH2OH | Cold water-soluble | Production of cellulose films | |||
| Hydroxypropyl methyl cellulose (HPMC) | Chloromethane and propylene oxide | −CH3 or −CH2CH(OH)CH3 | Cold water-soluble | Viscosity modifier, gelling, foaming and binding agent | E464 | ||
| Ethyl hydroxyethyl cellulose | Chloroethane and ethylene oxide | −CH2CH3 or −CH2CH2OH | E467 | ||||
| Carboxyalkyl | Halogenated carboxylic acids | Carboxymethyl cellulose (CMC) | Chloroacetic acid | −CH2COOH | Cold/Hot water-soluble | Often used as its sodium salt, sodium carboxymethyl cellulose (NaCMC) | E466 |
The sodium carboxymethyl cellulose can be cross-linked to give the croscarmellose sodium (E468) for use as a disintegrant in pharmaceutical formulations. Furthermore, by the covalent attachment of thiol groups to cellulose ethers such as sodium carboxymethyl cellulose, ethyl cellulose or hydroxyethyl cellulose mucoadhesive and permeation enhancing properties can be introduced.[64][65][66] Thiolated cellulose derivatives (see thiomers) exhibit also high binding properties for metal ions.[67][68]
Commercial applications
[edit]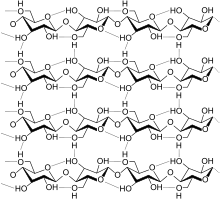
Cellulose for industrial use is mainly obtained from wood pulp and from cotton.[6]
- Paper products: Cellulose is the major constituent of paper, paperboard, and card stock. Electrical insulation paper: Cellulose is used in diverse forms as insulation in transformers, cables, and other electrical equipment.[69]
- Fibres: Cellulose is the main ingredient of textiles. Cotton and synthetics (nylons) each have about 40% market by volume. Other plant fibres (jute, sisal, hemp) represent about 20% of the market. Rayon, cellophane and other "regenerated cellulose fibres" are a small portion (5%).
- Consumables: Microcrystalline cellulose (E460i) and powdered cellulose (E460ii) are used as inactive fillers in drug tablets[70] and a wide range of soluble cellulose derivatives, E numbers E461 to E469, are used as emulsifiers, thickeners and stabilizers in processed foods. Cellulose powder is, for example, used in processed cheese to prevent caking inside the package. Cellulose occurs naturally in some foods and is an additive in manufactured foods, contributing an indigestible component used for texture and bulk, potentially aiding in defecation.[71]
- Building material: Hydroxyl bonding of cellulose in water produces a sprayable, moldable material as an alternative to the use of plastics and resins. The recyclable material can be made water- and fire-resistant. It provides sufficient strength for use as a building material.[72] Cellulose insulation made from recycled paper is becoming popular as an environmentally preferable material for building insulation. It can be treated with boric acid as a fire retardant.
- Miscellaneous: Cellulose can be converted into cellophane, a thin transparent film. It is the base material for the celluloid that was used for photographic and movie films until the mid-1930s. Cellulose is used to make water-soluble adhesives and binders such as methyl cellulose and carboxymethyl cellulose which are used in wallpaper paste. Cellulose is further used to make hydrophilic and highly absorbent sponges. Cellulose is the raw material in the manufacture of nitrocellulose (cellulose nitrate) which is used in smokeless gunpowder.
- Pharmaceuticals: Cellulose derivatives, such as microcrystalline cellulose (MCC), have the advantages of retaining water, being a stabilizer and thickening agent, and in reinforcement of drug tablets.[73]
Aspirational
[edit]Energy crops:
The major combustible component of non-food energy crops is cellulose, with lignin second. Non-food energy crops produce more usable energy than edible energy crops (which have a large starch component), but still compete with food crops for agricultural land and water resources.[74] Typical non-food energy crops include industrial hemp, switchgrass, Miscanthus, Salix (willow), and Populus (poplar) species. A strain of Clostridium bacteria found in zebra dung, can convert nearly any form of cellulose into butanol fuel.[75][76][77][78]
Another possible application is as Insect repellents.[79]
See also
[edit]- Gluconic acid
- Isosaccharinic acid, a degradation product of cellulose
- Lignin
- Zeoform
References
[edit]- ^ Nishiyama Y, Langan P, Chanzy H (2002). "Crystal Structure and Hydrogen-Bonding System in Cellulose Iβ from Synchrotron X-ray and Neutron Fiber Diffraction". J. Am. Chem. Soc. 124 (31): 9074–9082. doi:10.1021/ja0257319. PMID 12149011.
- ^ a b c d NIOSH Pocket Guide to Chemical Hazards. "#0110". National Institute for Occupational Safety and Health (NIOSH).
- ^ a b Crawford, R. L. (1981). Lignin biodegradation and transformation. New York: John Wiley and Sons. ISBN 978-0-471-05743-7.
- ^ Updegraff D. M. (1969). "Semimicro determination of cellulose in biological materials". Analytical Biochemistry. 32 (3): 420–424. doi:10.1016/S0003-2697(69)80009-6. PMID 5361396.
- ^ Romeo T (2008). Bacterial biofilms. Berlin: Springer. pp. 258–263. ISBN 978-3-540-75418-3.
- ^ a b c d e Klemm D, Heublein, Brigitte, Fink, Hans-Peter, Bohn, Andreas (2005). "Cellulose: Fascinating Biopolymer and Sustainable Raw Material". Angew. Chem. Int. Ed. 44 (22): 3358–3393. doi:10.1002/anie.200460587. PMID 15861454.
- ^ Cellulose. (2008). In Encyclopædia Britannica. Retrieved January 11, 2008, from Encyclopædia Britannica Online.
- ^ Chemical Composition of Wood. Archived October 13, 2018, at the Wayback Machine. ipst.gatech.edu.
- ^ Piotrowski, Stephan and Carus, Michael (May 2011) Multi-criteria evaluation of lignocellulosic niche crops for use in biorefinery processes Archived April 3, 2021, at the Wayback Machine. nova-Institut GmbH, Hürth, Germany.
- ^ Mantanis GI, Young RA, Rowell RM (1995). "Swelling of compressed cellulose fiber webs in organic liquids". Cellulose. 2 (1): 1–22. doi:10.1007/BF00812768. ISSN 0969-0239.
- ^ Payen, A. (1838) "Mémoire sur la composition du tissu propre des plantes et du ligneux" (Memoir on the composition of the tissue of plants and of woody [material]), Comptes rendus, vol. 7, pp. 1052–1056. Payen added appendices to this paper on December 24, 1838 (see: Comptes rendus, vol. 8, p. 169 (1839)) and on February 4, 1839 (see: Comptes rendus, vol. 9, p. 149 (1839)). A committee of the French Academy of Sciences reviewed Payen's findings in : Jean-Baptiste Dumas (1839) "Rapport sur un mémoire de M. Payen, reltes rendus, vol. 8, pp. 51–53. In this report, the word "cellulose" is coined and author points out the similarity between the empirical formula of cellulose and that of "dextrine" (starch). The above articles are reprinted in: Brongniart and Guillemin, eds., Annales des sciences naturelles ..., 2nd series, vol. 11 (Paris, France: Crochard et Cie., 1839), [ https://books.google.com/books?id=VDRsFWwgUo4C&pg=PA21 pp. 21–31].
- ^ Young R (1986). Cellulose structure modification and hydrolysis. New York: Wiley. ISBN 978-0-471-82761-0.
- ^ Kobayashi S, Kashiwa, Keita, Shimada, Junji, Kawasaki, Tatsuya, Shoda, Shin-ichiro (1992). "Enzymatic polymerization: The first in vitro synthesis of cellulose via nonbiosynthetic path catalyzed by cellulase". Makromolekulare Chemie. Macromolecular Symposia. 54–55 (1): 509–518. doi:10.1002/masy.19920540138.
- ^ Bishop, Charles A., ed. (2007). Vacuum deposition onto webs, films, and foils. Elsevier Science. p. 165. ISBN 978-0-8155-1535-7.
- ^ Dauenhauer P, Krumm C, Pfaendtner J (2016). "Millisecond Pulsed Films Unify the Mechanisms of Cellulose Fragmentation". Chemistry of Materials. 28 (1): 0001. doi:10.1021/acs.chemmater.6b00580. OSTI 1865816.
- ^ Wymer CE (1994). "Ethanol from lignocellulosic biomass: Technology, economics, and opportunities". Bioresource Technology. 50 (1): 5. Bibcode:1994BiTec..50....3W. doi:10.1016/0960-8524(94)90214-3.
- ^ Bidhendi AJ, Geitmann A (January 2016). "Relating the mechanical properties of the primary plant cell wall" (PDF). Journal of Experimental Botany. 67 (2): 449–461. doi:10.1093/jxb/erv535. PMID 26689854. Archived (PDF) from the original on January 13, 2018.
- ^ Bidhendi AJ, Chebli Y, Geitmann A (May 2020). "Fluorescence Visualization of Cellulose and Pectin in the Primary Plant Cell Wall". Journal of Microscopy. 278 (3): 164–181. doi:10.1111/jmi.12895. PMID 32270489. S2CID 215619998.
- ^ Deguchi S, Tsujii K, Horikoshi K (2006). "Cooking cellulose in hot and compressed water". Chemical Communications (31): 3293–5. doi:10.1039/b605812d. PMID 16883414.
- ^ Structure and morphology of cellulose Archived April 26, 2009, at the Wayback Machine by Serge Pérez and William Mackie, CERMAV-CNRS, 2001. Chapter IV.
- ^ Chen H (2014). "Chemical Composition and Structure of Natural Lignocellulose". Biotechnology of Lignocellulose: Theory and Practice (PDF). Dordrecht: Springer. pp. 25–71. ISBN 978-94-007-6897-0. Archived (PDF) from the original on December 13, 2016.
- ^ Mantanis GI (February 1, 2011). "PhD Thesis: Swelling of lignocellulosic materials in water and organic liquids; George Mantanis (Univ. of Wisconsin-Madison, 1994)". Academia.edu. Retrieved November 21, 2024.
- ^ Saito T, Kimura S, Nishiyama Y, Isogai A (August 2007). "Cellulose Nanofibers Prepared by TEMPO-Mediated Oxidation of Native Cellulose". Biomacromolecules. 8 (8): 2485–2491. doi:10.1021/bm0703970. PMID 17630692. Archived from the original on April 7, 2020. Retrieved April 7, 2020.
- ^ Peng, B. L., Dhar, N., Liu, H. L., Tam, K. C. (2011). "Chemistry and applications of nanocrystalline cellulose and its derivatives: A nanotechnology perspective" (PDF). The Canadian Journal of Chemical Engineering. 89 (5): 1191–1206. doi:10.1002/cjce.20554. Archived from the original (PDF) on October 24, 2016. Retrieved August 28, 2012.
- ^ Revol JF, Bradford H, Giasson J, Marchessault R, Gray D (June 1992). "Helicoidal self-ordering of cellulose microfibrils in aqueous suspension". International Journal of Biological Macromolecules. 14 (3): 170–172. doi:10.1016/S0141-8130(05)80008-X. PMID 1390450. Archived from the original on April 7, 2020. Retrieved April 7, 2020.
- ^ De France KJ, Hoare T, Cranston ED (April 26, 2017). "Review of Hydrogels and Aerogels Containing Nanocellulose". Chemistry of Materials. 29 (11): 4609–4631. doi:10.1021/acs.chemmater.7b00531.
- ^ Pranger L, Tannenbaum R (2008). "Biobased Nanocomposites Prepared by in Situ Polymerization of Furfuryl Alcohol with Cellulose Whiskers or Montmorillonite Clay". Macromolecules. 41 (22): 8682–8687. Bibcode:2008MaMol..41.8682P. doi:10.1021/ma8020213. Archived from the original on December 30, 2023. Retrieved June 19, 2023.
- ^ Kalashnikova I, Bizot H, Cathala B, Capron I (June 21, 2011). "New Pickering Emulsions Stabilized by Bacterial Cellulose Nanocrystals". Langmuir. 27 (12): 7471–7479. doi:10.1021/la200971f. PMID 21604688.
- ^ Kimura S, Laosinchai W, Itoh T, Cui X, Linder CR, Brown Jr RM (1999). "Immunogold labeling of rosette terminal cellulose-synthesizing complexes in the vascular plant vigna angularis". The Plant Cell. 11 (11): 2075–86. doi:10.2307/3871010. JSTOR 3871010. PMC 144118. PMID 10559435.
- ^ Taylor NG (2003). "Interactions among three distinct CesA proteins essential for cellulose synthesis". Proceedings of the National Academy of Sciences. 100 (3): 1450–1455. Bibcode:2003PNAS..100.1450T. doi:10.1073/pnas.0337628100. PMC 298793. PMID 12538856.
- ^ Richmond TA, Somerville CR (October 2000). "The Cellulose Synthase Superfamily". Plant Physiology. 124 (2): 495–498. doi:10.1104/pp.124.2.495. PMC 1539280. PMID 11027699.
- ^ a b Omadjela O, Narahari A, Strumillo J, Mélida H, Mazur O, Bulone V, et al. (October 29, 2013). "BcsA and BcsB form the catalytically active core of bacterial cellulose synthase sufficient for in vitro cellulose synthesis". Proceedings of the National Academy of Sciences of the United States of America. 110 (44): 17856–61. Bibcode:2013PNAS..11017856O. doi:10.1073/pnas.1314063110. PMC 3816479. PMID 24127606.
- ^ Popper ZA, Michel G, Hervé C, Domozych DS, Willats WG, Tuohy MG, et al. (2011). "Evolution and diversity of plant cell walls: from algae to flowering plants". Annual Review of Plant Biology. 62: 567–90. doi:10.1146/annurev-arplant-042110-103809. hdl:10379/6762. PMID 21351878. S2CID 11961888.
- ^ Peng L, Kawagoe Y, Hogan P, Delmer D (2002). "Sitosterol-beta-glucoside as primer for cellulose synthesis in plants". Science. 295 (5552): 147–50. Bibcode:2002Sci...295..147P. doi:10.1126/science.1064281. PMID 11778054. S2CID 83564483.
- ^ Endean, R (1961). "The Test of the Ascidian, Phallusia mammillata" (PDF). Quarterly Journal of Microscopical Science. 102 (1): 107–117. Archived (PDF) from the original on October 26, 2014.
- ^ Barkalow, David G., Whistler, Roy L. (2014). "Cellulose". AccessScience. doi:10.1036/1097-8542.118200.
- ^ Ignatyev I, Doorslaer, Charlie Van, Mertens, Pascal G.N., Binnemans, Koen, Vos, Dirk. E. de (2011). "Synthesis of glucose esters from cellulose in ionic liquids". Holzforschung. 66 (4): 417–425. doi:10.1515/hf.2011.161. S2CID 101737591. Archived from the original on August 30, 2017. Retrieved August 30, 2017.
- ^ La Reau A, Suen G (2018). "The Ruminococci: key symbionts of the gut ecosystem". Journal of Microbiology. 56 (3): 199–208. doi:10.1007/s12275-018-8024-4. PMID 29492877. S2CID 3578123.
- ^ Bowen R. "Digestive Function of Horses". www.vivo.colostate.edu. Archived from the original on November 12, 2020. Retrieved September 25, 2020.
- ^ Tokuda G, Watanabe H (June 22, 2007). "Hidden cellulases in termites: revision of an old hypothesis". Biology Letters. 3 (3): 336–339. doi:10.1098/rsbl.2007.0073. PMC 2464699. PMID 17374589.
- ^ Payne CM, Knott BC, Mayes HB, Hansson H, Himmel ME, Sandgren M, et al. (2015). "Fungal Cellulases". Chemical Reviews. 115 (3): 1308–1448. doi:10.1021/cr500351c. PMID 25629559.
- ^ Mettler, Matthew S., Vlachos, Dionisios G., Dauenhauer, Paul J. (2012). "Top Ten Fundamental Challenges of Biomass Pyrolysis for Biofuels". Energy & Environmental Science. 5 (7): 7797. doi:10.1039/C2EE21679E.
- ^ Czernik S, Bridgwater AV (2004). "Overview of Applications of Biomass Fast Pyrolysis Oil". Energy & Fuels. 18 (2). Energy & Fuels, American Chemical Society: 590–598. doi:10.1021/ef034067u. S2CID 49332510.
- ^ Dauenhauer PJ, Colby JL, Balonek CM, Suszynski WJ, Schmidt LD (2009). "Reactive Boiling of Cellulose for Integrated Catalysis through an Intermediate Liquid". Green Chemistry. 11 (10): 1555. doi:10.1039/B915068B. S2CID 96567659.
- ^ Teixeira AR, Mooney KG, Kruger JS, Williams CL, Suszynski WJ, Schmidt LD, et al. (2011). "Aerosol Generation by Reactive Boiling Ejection of Molten Cellulose". Energy & Environmental Science. 4 (10). Energy & Environmental Science, Royal Society of Chemistry: 4306. doi:10.1039/C1EE01876K. Archived from the original on August 31, 2017. Retrieved August 30, 2017.
- ^ Mettler MS, Mushrif SH, Paulsen AD, Javadekar AD, Vlachos DG, Dauenhauer PJ (2012). "Revealing pyrolysis chemistry for biofuels production: Conversion of cellulose to furans and small oxygenates". Energy Environ. Sci. 5: 5414–5424. doi:10.1039/C1EE02743C. Archived from the original on August 31, 2017. Retrieved August 30, 2017.
- ^ Mettler MS, Paulsen AD, Vlachos DG, Dauenhauer PJ (2012). "Pyrolytic Conversion of Cellulose to Fuels: Levoglucosan Deoxygenation via Elimination and Cyclization within Molten Biomass". Energy & Environmental Science. 5 (7): 7864. doi:10.1039/C2EE21305B.
- ^ Gibson LJ (2013). "The hierarchical structure and mechanics of plant materials". Journal of the Royal Society Interface. 9 (76): 2749–2766. doi:10.1098/rsif.2012.0341. PMC 3479918. PMID 22874093.
- ^ Stenius P (2000). "Ch. 1". Forest Products Chemistry. Papermaking Science and Technology. Vol. 3. Finland: Fapet OY. p. 35. ISBN 978-952-5216-03-5.
- ^ Wang H, Gurau G, Rogers RD (2012). "Ionic liquid processing of cellulose". Chemical Society Reviews. 41 (4): 1519–37. doi:10.1039/C2CS15311D. PMID 22266483.
- ^ a b c d Abetz V (2005). Encyclopedia of polymer science and technology (Wird aktualisiert. ed.). [Hoboken, N.J.]: Wiley-Interscience. ISBN 978-0-471-44026-0.
- ^ Woodings C (2001). Regenerated cellulose fibres. [Manchester]: The Textile Institute. ISBN 978-1-85573-459-3.
- ^ a b Borbély É (2008). "Lyocell, the New Generation of Regenerated Cellulose". Acta Polytechnica Hungarica. 5 (3).
- ^ "Cellulose Ether". methylcellulose.net. March 5, 2023. Archived from the original on March 7, 2023. Retrieved March 7, 2023.
- ^ "Methyl Cellulose". kimacellulose.com. Archived from the original on April 15, 2023. Retrieved April 15, 2023.
- ^ Maita P (2023). "Toward Sustainable Electronics: Exploiting the Potential of a Biodegradable Cellulose Blend for Photolithographic Processes and Eco-Friendly Devices". Advanced Materials Technologies. 1 (9). doi:10.1002/admt.202301282. hdl:2108/345525.
- ^ Lamanna L, Corigliano G, Narayanan A, Villani S, Friuli M, Chietera FP, et al. (October 15, 2024). "Beyond Plastic: Oleogel as gel-state biodegradable thermoplastics". Chemical Engineering Journal. 498: 154988. doi:10.1016/j.cej.2024.154988. hdl:10281/512761. ISSN 1385-8947.
- ^ Orlanducci P (2022). "Engineered surface for high performance electrodes on paper". Applied Surface Science. 608. doi:10.1016/j.apsusc.2022.155117.
- ^ Maita P (2023). "Toward Sustainable Electronics: Exploiting the Potential of a Biodegradable Cellulose Blend for Photolithographic Processes and Eco-Friendly Devices". Advanced Materials Technologies. 1 (9). doi:10.1002/admt.202301282. hdl:2108/345525.
- ^ Orlanducci P (2022). "Engineered surface for high performance electrodes on paper". Applied Surface Science. 608. doi:10.1016/j.apsusc.2022.155117.
- ^ Orlanducci P (2022). "A Sustainable Hydroxypropyl Cellulose-Nanodiamond Composite for Flexible Electronic Applications". Gels. 12 (8): 783. doi:10.3390/gels8120783. PMC 9777684. PMID 36547307.
- ^ Orlanducci P (2022). "Nanodiamond composites: A new material for the preservation of parchment". Journal of Applied Polymer Science. 32 (139). doi:10.1002/app.52742. S2CID 249654979.
- ^ Brunetti P (2020). "Nanodiamond-Based Separators for Supercapacitors Realized on Paper Substrates". Energy Technology. 6 (8). doi:10.1002/ente.201901233.
- ^ Clausen A, Bernkop-Schnürch A (2001). "Thiolated carboxymethylcellulose: in vitro evaluation of its permeation enhancing effect on peptide drugs". Eur J Pharm Biopharm. 51 (1): 25–32. doi:10.1016/s0939-6411(00)00130-2. PMID 11154900.
- ^ Rahmat D, Devina C (2022). "Synthesis and characterization of a cationic thiomer based on ethyl cellulose for realization of mucoadhesive tablets and nanoparticles". International Journal of Nanomedicine. 17: 2321–2334. doi:10.2147/IJN.S321467. PMC 9130100. PMID 35645561. S2CID 248952610.
- ^ Leonaviciute G, Bonengel S, Mahmood A, Ahmad Idrees M, Bernkop-Schnürch A (2016). "S-protected thiolated hydroxyethyl cellulose (HEC): Novel mucoadhesive excipient with improved stability". Carbohydr Polym. 144: 514–521. doi:10.1016/j.carbpol.2016.02.075. PMID 27083843.
- ^ Leichner C, Jelkmann M, Bernkop-Schnürch A (2019). "Thiolated polymers: Bioinspired polymers utilizing one of the most important bridging structures in nature". Advanced Drug Delivery Reviews. 151–152: 191–221. doi:10.1016/j.addr.2019.04.007. PMID 31028759. S2CID 135464452.
- ^ Seidi F, Saeb MR, Huang Y, Akbari A, Xiao H (2021). "Thiomers of chitosan and cellulose: Effective biosorbents for detection, removal and recovery of metal ions from aqueous medium". The Chemical Records. 21–152 (7): 1876–1896. doi:10.1002/tcr.202100068. PMID 34101343. S2CID 235368517.
- ^ Kohman, GT (July 1939). "Cellulose as an insulating material". Industrial and Engineering Chemistry. 31 (7): 807–817. doi:10.1021/ie50355a005.
- ^ Weiner ML, Kotkoskie, Lois A. (2000). Excipient Toxicity and Safety. New York: Dekker. p. 210. ISBN 978-0-8247-8210-8.
- ^ Dhingra D, Michael M, Rajput H, Patil RT (2011). "Dietary fibre in foods: A review". Journal of Food Science and Technology. 49 (3): 255–266. doi:10.1007/s13197-011-0365-5. PMC 3614039. PMID 23729846.
- ^ "Zeoform: The eco-friendly building material of the future?". Gizmag.com. August 30, 2013. Archived from the original on October 28, 2013. Retrieved August 30, 2013.
- ^ Thoorens G, Krier F, Leclercq B, Carlin B, Evrard B (2014). "Microcrystalline cellulose, a direct compression binder in a quality by design environment--a review". International Journal of Pharmaceutics. 473 (1–2): 64–72. doi:10.1016/j.ijpharm.2014.06.055. PMID 24993785.
- ^ Holt-Gimenez, Eric (2007). Biofuels: Myths of the Agrofuels Transition. Backgrounder. Institute for Food and Development Policy, Oakland, CA. 13:2 Holt-Giménez E. "Biofuels - Myths of the Agrofuels Transition: Parts I & II". Archived from the original on November 16, 2009. Retrieved September 5, 2013. Holt-Giménez E (November 13, 2009). "Biofuels - Myths of the Agrofuels Transition: Parts I & II". Archived from the original on September 6, 2013. Retrieved September 5, 2013.
- ^ MullinD, Velankar H.2012.Isolated bacteria, methods for use, and methods for isolation.World patent WO 2012/021678 A2
- ^ Sampa Maiti, et al. (December 10, 2015). "Quest for sustainable bio-production and recovery of butanol as a promising solution to fossil fuel". Energy Research. 40 (4): 411–438. doi:10.1002/er.3458. S2CID 101240621.
- ^ Hobgood Ray, Kathryn (August 25, 2011). "Cars Could Run on Recycled Newspaper, Tulane Scientists Say". Tulane University news webpage. Tulane University. Archived from the original on October 21, 2014. Retrieved March 14, 2012.
- ^ Balbo, Laurie (January 29, 2012). "Put a Zebra in Your Tank: A Chemical Crapshoot?". Greenprophet.com. Archived from the original on February 13, 2013. Retrieved November 17, 2012.
- ^ Thompson B (April 13, 2023). "Natural treatment could make you almost invisible to mosquito bites". New Atlas. Archived from the original on April 17, 2023. Retrieved April 17, 2023.
External links
[edit]- . Encyclopædia Britannica. Vol. 5 (11th ed.). 1911.
- Structure and morphology of cellulose by Serge Pérez and William Mackie, CERMAV-CNRS
- Cellulose, by Martin Chaplin, London South Bank University
- Clear description of a cellulose assay method at the Cotton Fiber Biosciences unit of the USDA.
- Cellulose films could provide flapping wings and cheap artificial muscles for robots – TechnologyReview.com

